Periodic Table Groups 1 8
Periodic table groups 1 8;
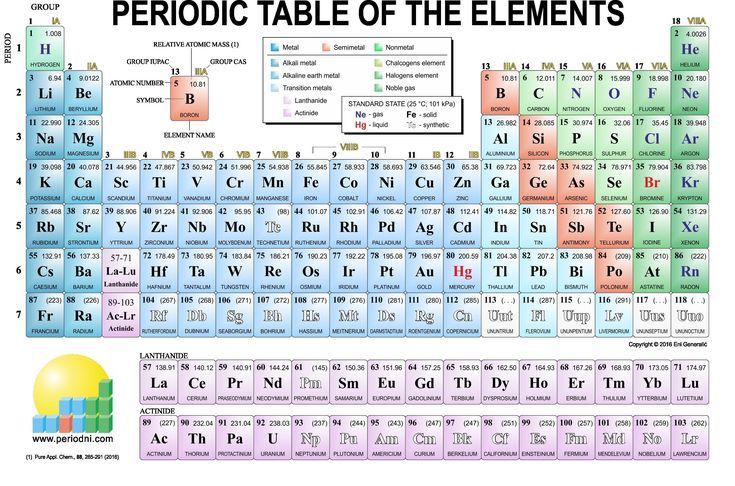
Periodic table groups 1 8. Elements in the same groups have the same number of valence electrons in the outer energy level. Groups and periods the periodic table is a way of arranging the elements so patterns in their properties and reactions can be identified and explained. The columns represent the groups.
The main group elements of the periodic table are groups 1, 2 and 13 through 18. Groups 1, 2 and 13 to 18 constitute the main group. Summary of the structure of the periodic table.
The group number is an identifier used to describe the column of the standard periodic table in which the element appears. The periodic table • in 1869,dmitri ivanovitch mendeléev created the first accepted version of the periodic table. Three systems have been used to number families and groups:
All the metals react : Terms in this set (44) 1. Periodic table groups and families;
These groups contain the most naturally abundant elements, comprise 80 percent of the earth's crust and are the most important for life. As a result, elements in the same group often display similar properties and reactivity. Groups groups run vertically in the periodic table.
Sulphur, which can form two single bonds to itself, comes in various ring sizes up to s 20, s 8 being the most stable. A group is any column on the periodic table. There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered.



















