Periodic Table Groups Definition
As a result, elements in the same group often display similar properties and reactivity.
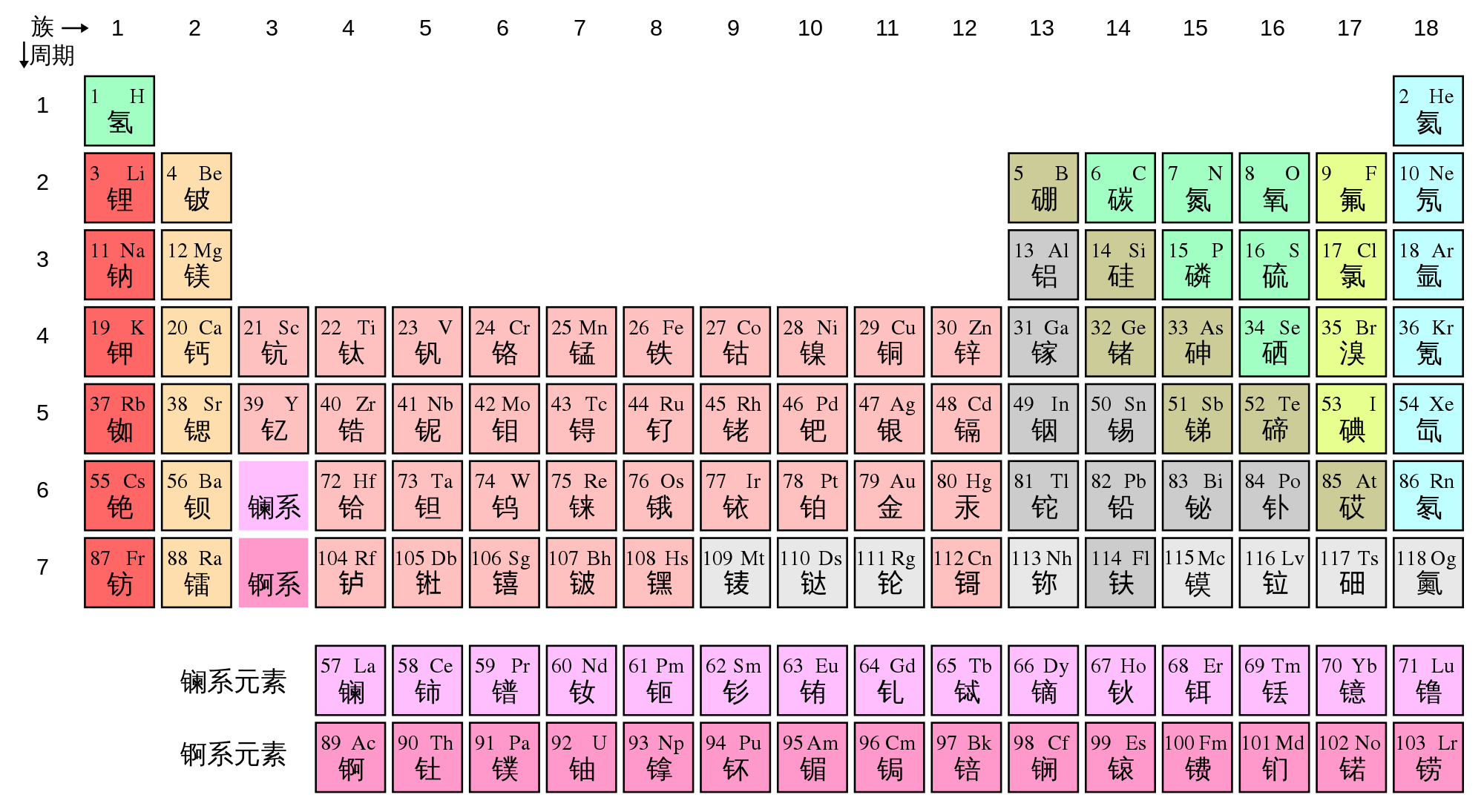
Periodic table groups definition. Group, in chemistry, a column in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; The modern periodic table is based on the modern periodic law put forward by the english physicist henry moseley, which states that “the properties of.
Groups are given a number to show where they are in the periodic table and also to identify the group of elements in them. A table in which the chemical elements are arranged in order of increasing atomic number. This is what is meant by periodicity or periodic table trends.
Elements with similar properties are arranged in the same column (called a group), and elements with the same number of electron shells are arranged in the same row (called a period). Note you can find many more hd periodic tables in our printable table collection.click the link for the specific periodic table if you want a black background, other color options, or the pdf version of the table. Elements in the same column have the same number of electrons in their outer shell (the highest energy level).
The noble gases are very unreactive. They all have a full outer shell of electrons, making them very stable (they tend not. Elements with similar properties are arranged in the same column (called a group), and elements with the same number of electron shells are arranged in the same row (called a period).
Flip through this interactive periodic table of elements. There are 18 columns or groups and different groups have different properties. Periodic table groups are columns of elements found in the modern periodic table.
The elements in each group have the same number of valence electrons. One example of a group is the noble or inert gases. There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered.



















